This is the cover of PDF on the solution of Past questions on ether. You can have this material at the bottom of the blog. Chapter ether is short and easy to understand.
My personal recommendation is to download notes on ether and attempts each and every question of PDF of a collection of Past Questions on it.
Very Short Answer Question:
1)What is the functional group of ether and amide?
The functional group of ether is –O- and amide is –CONH2.
2)Write the structure of:
Anisole and
2-methoxypropane.
The structure of anisole is:
The structure of 2-methoxypropane is
3)Write IUPAC names of ethers represented by molecular formula C5H12O.
4)Write down the possible unsymmetrical ethers of C4H10O and their IUPAC names.
The possible unsymmetrical ethers of C4H10O with their IUPAC name are:
5)There are three possible isomeric ethers of C4H10O. One of them is ethoxyethane. Write other two isomers and give their IUPAC name.
Other two isomers with their IUPAC name are:
6)What is unsymmetrical ether? Write an example and IUPAC name.
Those ether which have different groups across “O” atom are called
Unsymmetrical Ether. An example of Unsymmetrical ether is:
7)Write an example of Williamson’s etherification reaction. What is its importance?
Williamson's etherification reaction is important in the preparation of Unsymmetrical ether.
8)What is Williamson’s etherification reaction?
When haloalkane is heated with Sodium alkoxide, Ether is formed.
This reaction is called Williamson’s Etherification Reaction.
8)Prepare CH3-O-CH2CH3 by using Williamson’s ether synthesis.
CH3-O-CH2CH3 is prepared by using Williamson’s ether synthesis as:
9)What is unsymmetrical ether? Give Williamson’s synthesis to prepare unsymmetrical ethers.
Those ether which has different groups across “O” atom are called
Unsymmetrical Ether. For example:
Williamson’s synthesis to prepare unsymmetrical ethers is:
10)Write an unsymmetrical ether of C3H8O. How would you prepare the ether by using Williamson’s synthesis?
An unsymmetrical ether of C3H8O is:
And this ether is prepared by Williamson’s synthesis as:
11)How is unsymmetrical ether prepared by Williamson’s ether synthesis?
Unsymmetrical ether is prepared by Williamson’s ether synthesis by heating haloalkane and sodium alkoxide with different alkyl groups.
12)Give an example of unsymmetrical ether and write Williamson’s synthesis process for its preparation.
An example of unsymmetrical ether is:
Williamson’s synthesis process for its preparation is:
13)Write down isomeric ether of isopropyl alcohol and use Williamson’s synthesis process for the preparation of such ether.
Isomeric ether of isopropyl alcohol is:
Williamson’s synthesis process for the preparation of such ether is:
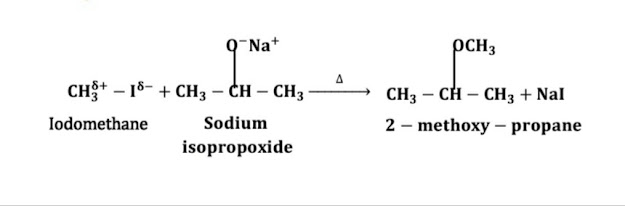
14)Write IUPAC name of CH3OCH(CH3)2 and use Williamson’s synthesis method for its preparation.
IUPAC name of CH3OCH(CH3)2 is 2-methoxy propane and Williamson’s synthesis method for its preparation is:
15)Give correct chemical reaction for the preparation of 2-methoxypropane.
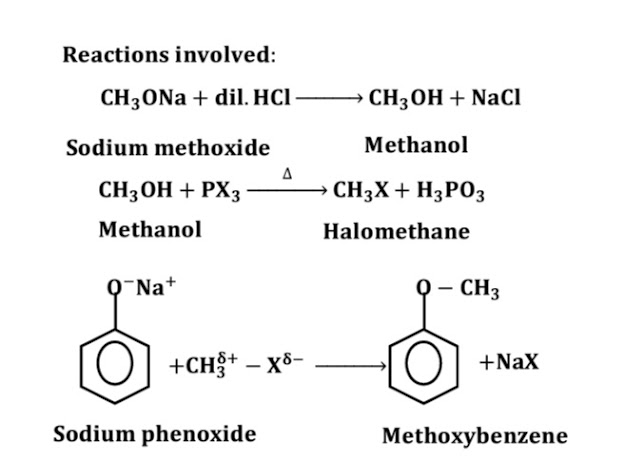
\16)What happens when Sodium phenolate reacts with iodomethane?
When Sodium phenolate reacts with iodomethane, anisole is formed.
17)Write the action of Sodium phenolate with iodomethane.
 18)Write chemical equation for the reactions taking place when:
18)Write chemical equation for the reactions taking place when:
Sodium phenoxide reacts with iodomethane.
19)What happens when Ethoxyethane is exposed to light and air?
When Ethoxyethane is exposed to light and air, diethyl peroxide is formed.
20)How would you obtain anisole from phenol?
Methoxybenzene is obtained from Phenol as:
1st Phenol is treated with sodium to form Sodium Phenoxide.
2nd Sodium phenoxide is treated with Methyl iodide to obtain Methoxy benzene
21)Give correct chemical reaction for the preparation of anisole.
22)Starting from CH3ONa, how would you prepare methoxybenzene?
23)What happens when methoxy benzene is treated with excess HI?
When methoxy benzene is treated with excess HI, Phenol and methyl chloride are formed.
24)Prepare methoxybenzene by using Willianson’s ether synthesis. (Answer same as 21)
25)How is methoxybenzene prepare from phenol? (Answer same as 21)
26)Give reason:
a)It is dangerous to boil sample of ether stored for a long time.
This is because there may be
thermally unstable diethyl peroxide that forms on reaction of ether with air in
the presence of sunlight.
b)Ether is stored in a bottle containing iron wire.
In case of ether stored in a bottle
containing iron gauge cap, Oxygen being more reactive iron than ether forms
Ferrous oxide preventing formation of diethylperoxide which explode with rise
in temperature.
27)Write down isomeric ether of isopropyl alcohol. What happens when the isomeric ether is heated with excess HI?
Isomeric ether of isopropyl alcohol is Methoxyethane
When Methoxyethane is heated with excess HI Iodomethane and Iodoethane are formed.
28)Write an example of Williamson’s etherification. Name the isomer of C2H6O which reacts with excess HI, gives iodomethane as major product and write reaction for it.
Answer of 1st part is same as 7.
Answer of second part:
The isomer of C2H6O which reacts with excess HI, gives iodomethane as major product is Methoxymethane and reaction is:
29)What is the action of ethoxyethane with
a)PCl5 and
b)HI (conc. and cold) (question wrong)
The action of ethoxyethane with PCl5 is:
30)What action takes place when excess of ethanol is heated with conc. Sulphuric acid at about 140°C?
When excess of ethanol is heated with conc. Sulphuric acid at about 140°C, Ethoxy ethane is formed.
31)Convert ethoxyethane into methoxy ethane. [2 times]
32)How would you obtain methoxyethane from ethanol?
33)Write a sequence of chemical reactions for the conversion of ethoxyethane to methoxyethane. (Answer is same as 30)
Short Answer Question:
1)Describe the laboratory method of preparation of ethoxyethane. How is ethoxyethane converted into methoxy methane?
In
lab ether is prepared by heating ethanol with concentrated sulphuric acid at 140°C.
Temperature
shouldn’t exceed
while lab preparation of ether as ethene forms
at 160-170°C.
Procedure:
Ethanol
and Conc.
are taken in 2:1 ration in round bottom flask.
Then this mixture is heated over sand bath at
. As a result, ether starts to form that passes
through eater condenser. Thus formed ether is collected in receiver held in
water bath with ice cubes. As soon as ether starts to form ethanol is added to
R.B flask through thistle funnel for continuous etherification. The resultant
mixture is transferred to separating funnel to separate ether from aqueous
layer. Then ether is subjected to purification.
Purification:
1. Ether is washed with NaOH solution to remove
acidic layer.
2. Ether then is washed with water
several times to remove water-soluble impurities.
3. Then, alcohol present it, is removed
with 50% Calcium chloride solution.
4. Then ether is dried over anhydrous
Calcium chloride
5. Finally, pure and dry ether is obtained
by distillation at 32-35°C
2)Describe the laboratory method of preparation of ethoxyethane. Why is it dangerous to boil old sample of ether?
Answer is same as 1
Answer of second part:
This is because there may be
thermally unstable diethyl peroxide that forms on reaction of ether with air in
the presence of sunlight.
3)Describe the laboratory method of preparation of ethoxyethane. What happens when ethoxyethane is exposed to air?
Answer of first part is same 1
Answer of second part is:
4)Write down method of preparation of ethoxyethane. Answer of first part is same as1.
5)Write down suitable chemical reaction for the preparation of ethoxyethane from ethanol. How is ethoxyethane converted into methoxy methane? What happens when ethoxyethane is heated with:
Air?
Conc. H2SO4? [3 times]
Answer of first part:
Answer of second part: 31 (Very Short answer question)
Answer of third part a) is same as
b)
6)How is ethoxyethane prepared in the laboratory in pure and dry state? (Answer is same as 1)
7)Describe laboratory method of preparation of ethoxyethane with neat and labeled diagram.(Answer is same as 1)
8)How is ethoxyethane prepared in the laboratory? Write Williamsons’ synthesis for the preparation of an unsymmetrical ether.
Answer of first part is same as 1
Answer of second part is the same as 10. (Very Short answer question)
9)Describe the preparation of ethoxyethane in the laboratory . ((Answer is same as 1))
10)Write short notes on Laboratory preparation of ethoxy ethane. [3 times] (Answer is same as 1)
11)Write short notes on Laboratory preparation of diethyl ether.(Answer is same as 1)
CLICK DOWNLOAD BUTTON TO ACCESS PDF ON SOLUTION OF PAST QUESTIONS ON ETHER







 18)Write chemical equation for the reactions taking place when:
18)Write chemical equation for the reactions taking place when:








































ConversionConversion EmoticonEmoticon