This is the cover of PDF on full note that will be published soon for download. Collection of Past questions and its solution will be the next to be published.
This chapter is central for Aromatic ABCD questions and conversion too. Lab preparation of Nitobenzene is most popular question and the reduction of nitrobenzene in a different medium is next.
NITROCOMPOUNDS
Introduction
Nitro
compounds those organic compounds which have nitro
( –NO2 )as a functional
group.
Ambident Group/ Nucleophile
Some functional groups can link to alkyl or aryl groups in two ways. Such groups are termed as the Ambident group.
Hence Ambident group can be defined as those groups having two linking sites. For example:
CN, –NO2 , etc
Illustration:
Why is CN an ambident group?
CN group is an ambident group/ nucleophile because it can link to an alkyl/ aryl group either through C atom forming cyanide or nitrile or through N atom forming isocyanide.
Why is NO2 an ambident group?
NO2 group is an ambident group/ nucleophile because it can link to an alkyl/ aryl group either through N atom forming nitro compounds or through O atom forming nitrite.
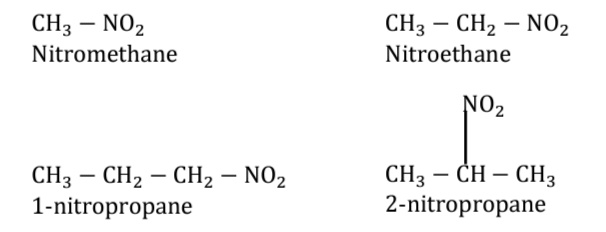
Classification Of Nitro compounds |
A A) Primary nitroalkane (1°nitroalkane):
Primary nitroalkane is that nitroalkane in which nitro ( –NO2 ) is attached to a primary
carbon atom. It is denoted by:
For
example:
B) Secondary nitroalkane (2°nitroalkane):
Secondary nitroalkane is that nitroalkane in which nitro ( –NO2 ) is attached to a secondary
carbon atom. It is denoted by:
C) Tertiary nitroalkane (3°nitroalkane):
Tertiary nitroalkane is that nitroalkane in which nitro ( –NO2 ) is attached to a tertiary
carbon atom. It is denoted by:
Isomerism
C3H7NO2
C4H9NO2
C5H11NO2
General method of preparation of Nitroalkane
From alkane:
When haloalkane is treated with fuming nitric acid, nitroalkane is formed. It follows a free radical mechanism so there is homolysis of both C-H and C-C bond of alkane resulting mixture of nitroalkanes.
From haloalkane:
When haloalkane is treated with alcoholic silver nitrate, nitroalkane is formed.
Laboratory Preparation Of Nitrobenzene
Principle: When benzene is heated with concentrated nitric acid in the presence of Conc. sulphuric acid at 60°C, nitrobenzene is formed.
The temperature shouldn't exceed 60° as 1,3-dinitrobenzene forms at higher temperatures.
Procedure:
To 120 ml of Conc. Sulphuric acid in a round bottom flask, 60 ml of Conc, nitric acid: 3ml at each time followed by constant stirring under cold water bath. The mixture so produced is called a nitrating mixture. To this, 70 ml of benzene is similarly added. The resultant mixture is refluxed at over water bath. Heating is continued until yellow liquid with bitter almond smell appeared. Then, the mixture formed is transferred to separating funnel to remove the nitrobenzene layer from an aqueous acidic layer.
Purification:
The nitrobenzene layer is washed with NaOH solution to remove acidic impurities.
Then it is washed with water several times so as to remove water-soluble impurities.
Nitrobenzene is then dried over anhydrous Calcium chloride.
Finally pure and dry nitrobenzene is obtained by distillation at 208-212°C
Physical Properties
State:
Nitroalkanes are liquid whereas, aromatic nitro compounds are liquid or solid.
Color:
Nitroalkanes are colorless whereas, aromatic nitro compounds are pale yellow.
Odor:
Nitroalkanes are pleasant to smell whereas, aromatic nitro compounds have a characteristic smell.
Boiling Poing:
Nitro compounds have a high boiling point than hydrocarbon of comparable mass. This is due to strong dipole-dipole interactions.
Solubility:
Lower members are slightly soluble in water. Higher members are soluble in organic compounds.
Chemical properties
I)Reduction: The product of reduction depends upon the nature of the reducing agent.
A) Catalytic reduction:
When nitro compounds are heated with hydrogen in the presence of a metal catalyst (Ni, Pt Or Pd), corresponding amines are formed.
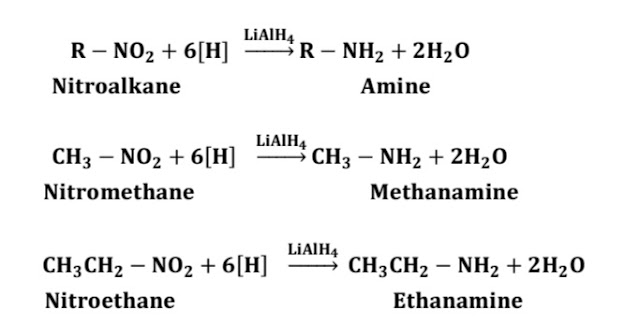
B) Reduction with metal hydride:
i) When nitroalkane is treated with Lithium Aluminium hydride (LiAlH4), corresponding amines are formed.
ii) When nitrobenzene is treated with (LiAlH4), azobenzene is formed.
C) Reduction with different medium:
i) In acidic medium: When nitro compounds are reduced with metal (Sn, Zn or Fe) in the presence of concentrated hydrochloric acid, corresponding amines are formed.
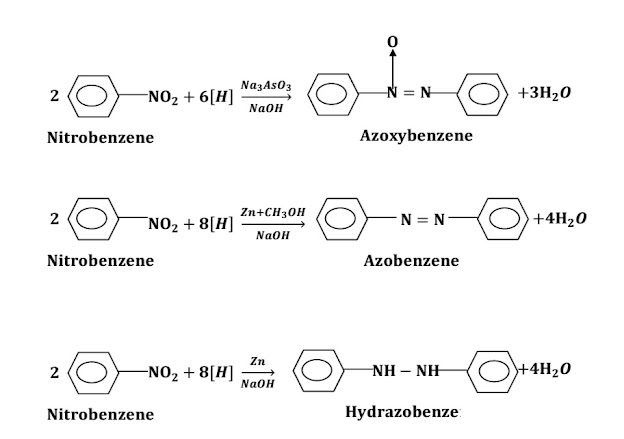
ii) In basic medium: Aliphatic amines do not undergo a reduction in basic medium but Nitrobenzene forms different products in basic medium.
a) Reduction with Na3AsO3+NaOH: When Nitrobenzene is treated with Na3AsO3+NaOH, Azoxybenzene is formed.
b) Reduction with Zn+CH3OH+NaOH: When Nitrobenzene is treated with Zn+CH3OH+NaOH, Azobenzene is formed.
c) Reduction with Zn+NaOH: When Nitrobenzene is treated with Zn/CH3OH+NaOH, Hydrazobenzene is formed.
iii) In neutral medium:. When Nitrobenzene is treated with Zn/NH4Cl or Zn/H2O, the corresponding Hydroxylamine is formed.
iv)Electrolytic reduction:
a)In the presence of weak acid, anilline is formed.
b)In the presence of strong acid, N-hydroxylamine is formed that rearrange to form p-aminophenol.
II) Electrophilic Substitution Reaction:
A) Reactivity:
i)
When nitrobenzene contains NO2 group which is -I group i.e. it withdraws electron density of ring making it less reactive than benzene toward electrophilic substitution reaction.
B) Orientation:
Due
to resonance in nitrobenzene, ortho and para positions of the ring get positive
charge indicating low electron density at these positions hence they repel
electrophile instead meta-position attracts it That is why the nitro group is meta
directing in an electrophilic substitution reaction.
i) Halogenation:
When benzene is treated with Chlorine in the presence of FeCl3, 3-nitro chlorobenzene is formed.
ii)Nitration:
When benzene is treated with fuming Nitric acid in the presence of Conc. H2SO4, 1,3-dinitrobenzene is formed.
iii) Sulphonation:
When benzene is treated with fuming Sulphuric acid 3-nitrobenzene sulphonic acid is formed.
Uses
As an oxidizing agent in organic synthesis.
For making shoe polishes.
For scenting cheap soaps.
For making aniline.
Dear Students It would take some time to upload PDF for download.
This site provides notes on different chapters of Chemistry of Grade XI and XII (NEB Board). Also, it posts number of blogs on Collection of past questions and solution. Conversion chart, rules, Chemical test are some additional features.




























ConversionConversion EmoticonEmoticon