It
is carried out in the following steps:
- 1 ml of iodine solution is taken in a clean and dry test tube.
- Then, NaOH solution is added till red color of the iodine solution disappears.
- To this, a small amount of organic sample is added.
- Then it is warmed at 60-70C.
- Hospital smell confirms the test is positive.
Primary
alcohol upon analysis, it can be concluded only Primary alcohol that gives
iodoform test is ethanol because all other primary alcohol lacks CH3-CH-OH
group.
Only
Secondary alcohol having –OH group at 2nd Carbon gives the iodoform
test. For example: 2-propanol, 2-butanol,etc.
None
of the tertiary alcohol gives the iodoform test.
Aldehyde
upon analysis, it can be concluded only Aldehyde that gives iodoform test is
ethanal as other aldehyde lacks CH3-CO- group.
No,
only some ketone gives the iodoform test. Ketone having CO group at second position
gives the iodoform test. For example: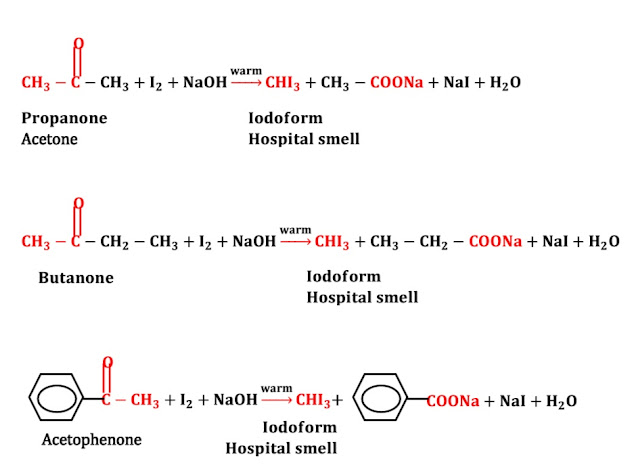
11) NAME TWO EXAMPLES OF SECONDARY ALCOHOL THAT GIVES IODOFORM TEST.
2-propanol
and 2-butanol are examples of two secondary alcohol that gives the iodoform test.
Yes,
iodoform test can be used as a test of ethanol. Esterification reaction and
KMnO4 test can also be used as its alternative.
14) CAN IODOFORM TEST BE USED TO DISTINGUISH TWO ALDEHYDE?
Yes,
Iodoform test be used to distinguish two aldehydes however, one aldehyde must
be ethanal (acetaldehyde).
15) CAN IODOFORM TEST BE USED TO DISTINGUISH TWO KETONE?
Yes,
Iodoform test be used to distinguish two ketones, however, only one ketone must have
CH3CO group.
16) CAN IODOFORM TEST BE USED TO DISTINGUISH Propanal & Propanone?
Yes, Iodoform test be used to distinguish Propanal and Propanone. Instead of this test, Tollen’s test or Fehling test can also be used.
17)2-propanol GIVES IODOFORM TEST BUT 1-propanol DOESNT GIVE THIS TEST, WHY?
2-propanol
gives iodoform test but 1-propanol doesn’t give this test. This is because
2-propanol has CH3CHOH group which oxidizes to CH3CO group. Whereas, 1-propanol
lacks such a group.
18)NAME THE ISOMER OF C3H6O THAT GIVES IODOFORM TEST.
Propanone
is the desired isomer of C3H6O that gives the iodoform test.
19)NAME THE ISOMER OF C4H8O THAT GIVES IODOFORM TEST.
Butanone
is the desired isomer of C4H8O that gives the iodoform test.
20)NAME THE ISOMER OF C5H10O THAT GIVES IODOFORM TEST.
2-pentanone and 3-methylbutanone are the desired isomer of C5H10O that gives the iodoform test.
21)NAME THE ISOMER OF C3H8O THAT GIVES IODOFORM TEST.
2-propanol is the desired isomer of C3H8O that gives the iodoform test.
22) CAN SODIUM CARBONATE BE USED INSTEAD OF SODIUM HYDROXIDE?
















ConversionConversion EmoticonEmoticon