Amines
Introduction
Amines are the alkyl or aryl derivatives of ammonia Or, Amines are those organic compounds formed which are formed by replacing one or more H-atoms of ammonia by Alkyl or aryl group. For examples:
Types Of amines
Depending
upon the number of H-atoms replaced by alkyl or aryl group, amines are of following
types:
Primary amines (1° amine)
Secondary amines (2° amine)
Tertiary amines (3° amine)
Primary amines (1° amine):
Those amines
which are formed by replacing one “H” atom by alkyl or aryl group are called
Primary amines (1° amine)
They are
denoted by:
Secondary amines (2°amines)
Those amines
which are formed by replacing two “H” atoms by alkyl or aryl groups are called
Secondary amines (2°amines).
They are
denoted by:
Tertiary amines (3°amines):
Those amines
which are formed by replacing three “H” atoms by alkyl or aryl group are called
Tertiary amines (3°amines).
They are denoted by:
For
examples:
Nomenclature
Common names:
Amines are
commonly named as alkyl amine. For examples:
If more than
one same alkyl group is present then di, tri…is added to the name of the alkyl group.
For example:
If different
kinds of the alkyl group are the present name of alkyl groups are placed in
alphabetical order. For examples:
IUPAC nomenclature:
In the case of
primary amines,
They are
named as Alkanamine. For examples:
Position of
NH2 is indicated from amines having 3 or more than 3 C atoms.
The name of the substituent is prefixed.
In the case of
secondary amine,
Longest
alkyl group qualifies for parent chain and hence Parent named becomes
alkanamine.
For example:
Parent name (Alkane) and another as substituent (alkyl) which is preceded by "N-"
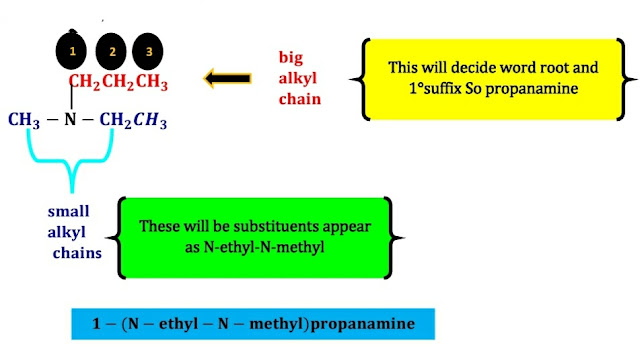
In the case of
tertiary amine,
Longest alkyl group qualifies for parent chain and hence Parent named becomes alkanamine.
Small two alkyl chain is treated as a substituent and added to parent name. The name of small alkyl group is prefixed by "N,N-".
Also, di is added before the name of the alkyl group
for the same two small alkyl groups.
If different
small alkyl groups are present then their names are placed in alphabetical
order each prefixed by "N-" For example.
For example:
Nomenclature of Aromatic amine:
Commonly they are named as aryl amine. Simplest aryl amine is Phenyl amine. i.e:
It is also
commonly known as aniline and all other simple arylamine is commonly named as
derivative of aniline.
IUPAC name
of aniline is Benzenamine and rest simple aryl amine is named as derivative of
Benzenamine.
Isomerism:
They exhibit
metamerism, Positional, and Chain isomerism:
Isomers of C3H9N
Isomers of C4H11N
Hoffmann’s separation of mixture
containing Primary, Secondary and Tertiary Amines
Primary
amine reacts with diethyloxalate thereby forming dialkyloxamide which is solid.
Secondary
amine reacts with diethyloxalate thereby forming dialkyloxamic ester which is
oily.
Tertiary
amine doesn’t react with diethyloxalate because it doesn’t have replaceable
hydrogen.
Resultant mixture is subjected to filtration. Dialkyl oxamide which is solid is obtained as residue. Where as rest of the components (dialkyl oxamic ester, ethanol, tertiary amine) appear in filtrate as they are in liquid state.
Primary amine is recovered from dialkyl oxamide by distillation with KOH.
Thus obtained filtrate is subjected to distillation. Tertiary amine being most volatile is obtained as first fraction followed by ethanol.
Dialkyl oxamic ester which is obtained as residue is subjected to distillation with KOH to recover Secondary amine.
In this way, mixture containing Primary, Secondary and Tertiary amine is separated by using Hoffmann's method.
GENERAL METHODS OF PREPARATION OF AMINES
1)From haloalkane:
Preparation of amines from haloalkane is not useful. This is not useful as mixture of amines forms.
2)From nitro compounds :
Nitro compounds on catalytic reduction or reduction in acidic medium forms amines.
3)From alkyl cyanide (Alkane nitrile )
On reduction of alkyl cyanide or Alkane nitrile, Primary amine is formed.
4) From alkyl isocyanide:
On reduction of alkyl isocyanide., Secondary amine is formed.
5) From amide
A)By reduction:
On reduction of amide, Primary amine is formed.
B) By decarbonylation (Hoffmann degradation reaction/Hoffmann hypobromite reaction)
When amide is warmed with bromine in the presence of alkali, amine is formed with one carbon less than corresponding amide. It is called decarbonyl reaction involved removal of CO group.
LABORATORY PREPARATION OF ANILINE
Principal
In lab, aniline is prepared by heating nitrobenzene with Tin in the presence of Conc.HCl.
Reaction in detail:
Procedure
50g of tin is taken in round bottom flask. To this, 25 g of nitro benzene is added. To this 100 ml of Conc HCl is added. Thus mixture is then refluxed at 90˚ over water bath. Heating is continued till yellow color with bitter almond smell disappears. To the resultant mixture, calculated amount of sodium hydroxide is added to obtain aniline from double salt phenyl ammonium chlorostannate.
The resultant mixture is subjected to steam distillation. Aniline being steam volatile, steam selectively removes aniline from the mixture resulting aqueous solution of aniline as distillate which is then subjected to purification.
Purification
To the aqueous solution of aniline, NaCl is added till saturation. Aniline layer separates out.
To this ether is added to obtain ethereal extract of aniline.
Then ethereal extract is stand at room temperature overnight to remove ether.
To this solid KOH is added to dehydrate aniline.
Finally pure and dry aniline is obtained by distillation at 182-183˚
C.
PHYSICAL PROPERTIES OF AMINES
CHEMICAL PROPERTIES OF AMINES
1)Basicity
Amines are basic in nature because of the lone pair of electrons present in Nitogen atom, hence amines are Lewis base as it can form coordinate complex with Lewis acid.
Also it is Bronsted base as it accepts protons. For example:
a)Comparison of basicity of aliphatic and aromatic amine (Aniline)
Aniline is weaker base than aliphatic amines as lone pair of electron at N atom is less available due to resonance.
Resonance doesn't occur in aliphatic amines. Also, it has alkyl group which is electron releasing group which increases basicity.
That is why, aniline is less basic than aliphatic amines.
b) Comparison of basicity of ammonia and aniline:
Aniline is weaker base than ammonia because lone pair of electron is less available at N-atom due to resonance.
Ammonia doesn't exhibit resonance. That why, ammonia is more basic than aniline.
c)Comparison of basicity of ammonia and aliphatic amines
Aliphatic Amines are stronger base than ammonia. This is because aliphatic amines have alkyl group which is electron releasing group that increase electron density at N atom which makes lone pair more available. Such group is not present in ammonia.
d)Comparison of basicity of primary, secondary and tertiary amines
Basicity of amines depend on two factors:
Inductive effect
Alkyl group is +I group i.e. it is electron releasing group. Such group increases electron density on N atom making species more basic. Higher the number of such groups, higher is basicity.
Solvation effect
Basicity of amines also depend upon extent of solvation of conjugate acid formed from amines. Conjugate acid from primary amine is most solvated as it can form higher number of H bonding with water. On contrary, conjugate acid of tertiary amine is least hydrated as it forms least number of H bonding with water.
Higher the solvation of conjugate acid, higher is its stability, higher the stability of conjugate acid stronger is the base.
Due to cumulative effect of inductive effect and solvation effect, the basicity follows following order:
2)Alkylation
When amine is heated with haloalkane, higher amines are formed. Alkylation continues till tetra alkyl ammonium halide forms.
3)Acylation
When amine is treated with acid chloride or acid anhydride, substituted amide is formed.
4)Reaction with Grignard reagent
When primary or secondary amines is treated with Grignard reagent, alkane is formed.
5)Reaction with nitrous acid
A)Primary amine reacts with nitrous acid thereby forming alcohol with the evolution of nitrogen gas.
B)Secondary reacts with nitrous acid thereby forming N-nitroso amine which gives violet color upon reaction with phenol and Conc. Sulphuric acid followed by addition of alkali.
C)Tertiary amine reacts with nitrous acid forming water soluble salt.
6)Reaction with chloroform/ Isocyanide test/ Carbylamine reaction
When primary amine is warmed with chloroform in the presence of alcoholic KOH or NaOH, corresponding isocyanide is formed which have offensive smell. This reaction is called Isocyanide reaction or Carbylamine reaction.
Reaction with benzene sulphonyl chloride (Hinsberg test)
A)Primary amine when treated with benzene sulphonyl chloride, N-alkylbenzene sulphonamide formes which is soluble in alkali.
B)Secondary amine when treated with benzene sulphonyl chloride, N, N-dialkylbenzene sulphonamide formes which is insoluble in alkali.
C) Tertiary amine doesn't react with benzene sulphonyl chloride because it lacks replaceable hydrogen.
Electrophilic substitution reaction
Reactivity
-NH2 is electron releasing group so it increases electron density of aromatic ring making aniline it more reactive than benzene in electrophilic substitution reaction.
Orientation
Due to resonance in aniline, ortho and para position bear negative charge making ortho and para are electron rich. Hence, these positions attract electrophile.
Thus -NH2 is ortho/ para directing towards electrophilic substitution reaction.
A) Halogenation:
Aniline when treated with aqueous bromine, 2, 4, 6-tribromo aniline is formed.
To prevent formation of polyhalo aniline, aniline is protected by acylation and deprotection by acid hydrolysis after halogenation.
B)Nitration
Nitrating mixture is an acid and aniline is base so protonation of aniline takes place resulting NH3+, which is meta directing so m-nitroaniline forms along with ortho and para.
Also, these are oxidising agent hence oxidation of aniline also competes with nitration.
To prevent these undesired reactions, -NH2 is protected.
C) Sulphonation
Aniline when heated with conc. Sulphuric acid at 185-200C, Sulphanilic acid is formed.
2) Oxidation
Aniline when treated with potassium dichromate, p-benzoquinone is formed.
3) Diazotization reaction
Aniline when treated with sodium nitrite in the presence of dilute hydrochloric acid at 0-5C, benzene diazonium chloride salt us formed.
Application of benzene diazonium chloride salt
A) Sandmayer reaction
Benzene diazonium chloride salt is treated with CuCl/HCl or CuBr/HBr, corresponding halobenzene is formed. This reaction is called Sandmayer reaction.
B) Gattermann reaction
Benzene diazonium chloride salt is treated with Cu/HCl or Cu/HBr, corresponding halobenzene is formed. This reaction is called Gattermann reaction.
C) Preparation of Phenol
Benzene diazonium chloride salt when is warmed with water or dilute sulphuric acid, phenol is formed.
D) Diazo-coupling reaction or Coupling reaction
Benzene diazonium chloride salt when treated with Phenol in tge presence of acid or aniline in the presence of base corresponding azodye is formed. This reaction is called coupling reaction or Diazo-coupling reaction.
Note:
Azo dyes are class of dyes having azo (-N=N-) as chromophore. For example:
p-aminoazobenzene & p-hydroxyazobenzene
Test of amines
6)Reaction with chloroform/ Isocyanide test/ Carbylamine reaction
When primary amine is warmed with chloroform in the presence of alcoholic KOH or NaOH, corresponding isocyanide is formed which have offensive smell. This reaction is called Isocyanide reaction or Carbylamine reaction.
5)Reaction with nitrous acid
A)Primary amine reacts with nitrous acid thereby forming alcohol with the evolution of nitrogen gas.
B)Secondary reacts with nitrous acid thereby forming N-nitroso amine which gives violet color upon reaction with phenol and Conc. Sulphuric acid followed by addition of alkali.
C)Tertiary amine reacts with nitrous acid forming water soluble salt.
Reaction with benzene sulphonyl chloride (Hinsberg test)
A)Primary amine when treated with benzene sulphonyl chloride, N-alkylbenzene sulphonamide formes which is soluble in alkali.
B)Secondary amine when treated with benzene sulphonyl chloride, N, N-dialkylbenzene sulphonamide formes which is insoluble in alkali.
C) Tertiary amine doesn't react with benzene sulphonyl chloride because it lacks replaceable hydrogen.








































































ConversionConversion EmoticonEmoticon